“CRISPR-Cas systems” in bacteria and viruses identify and destroy invading viral sequences. It is bacterial and archaeal immune system for protection against viral infections. In 2012, CRISPR-Cas system was recognised as a genoma, editing tool. Since then, wide range of CRISPR-Cas systems have been developed and have found applications in areas such as in gene therapy, diagnostics, research and crop improvement. However, currently available CRISPR-Cas systems have limited clinical use due to frequent occurrences of off-target editing, unexpected DNA mutations and inheritable problems. Researchers have recently reported a novel CRISPR-Cas system that can target and destroy mRNA and proteínas associated with different genetic diseases more accurately without off-target impact and inheritable problems. Named Craspase, it is the first CRISPR-Cas system that shows proteína editing function. It is also the first system that can edit both RNA and proteína. Because Craspase overcomes many limitations of existing CRISPR-Cas systems, it has potential to revolutionise gene therapy, diagnostics and monitoring, biomedical research, and crop improvement.
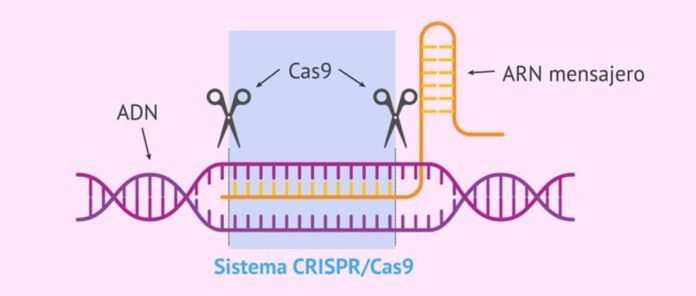
“CRISPR-Cas system” is natural immune system of bacteria and archaea against viral infections that identifies, binds and degrades the sequences in the viral gene to protect. It consists of two parts – bacterial RNA transcribed from the viral gene incorporated in the bacterial genome after first infection (called CRISPR, this identifies the target sequences of the invading viral genes) and an associated destroyer proteína called “CRISPR associated proteína (Cas)” which binds and degrades the identified sequences in the viral gene to protect the bacteria against viruses.
MÁS CRUJIENTE significa "repeticiones palindrómicas cortas agrupadas y regularmente espaciadas". Es ARN bacteriano transcrito caracterizado por repeticiones palindrómicas.
Las repeticiones palindrómicas (CRISPR) se descubrieron por primera vez en las secuencias de E. coli in 1987. In 1995, Francisco Mojica observed similar structures in archaea, and it was he who first thought of these as a part of the immune system of bacteria and archaea. In 2008, it was experimentally demonstrated for the first time that the target of the immune system of bacteria and archaea was foreign DNA and not mRNA. The mechanism of identification and degradation viral sequences suggested that such systems could be used as a tool for edición del genoma basado en CRISPR. Since its recognition as a genome editing tool in 2012, CRISPR–Cas system has come a very long way as a firmly established standard edición de genes system and has found a wide range of applications in biomedicine, agriculture, pharmaceutical industries including in clinical gene therapy1,2.
Una amplia gama de CRISPR-Cas systems are already identified and currently available for monitoring and editing DNA/RNA sequences for research, drug screening, diagnostics and treatments. The current CRISPR/Cas systems are divided into 2 classes (Class 1 and 2) and six types (Type I to XI). Class 1 systems have multiple Cas proteínas which need to form a functional complex to bind and act on their targets. On the other hand, Class 2 systems have only one large Cas proteína for binding and degrading target sequences which makes Class 2 systems easier to use. Commonly used Class 2 systems are Cas 9 Type II, Cas13 Type VI, and Cas12 Type V. These systems may have undesired collateral effects I.e., off-target impact and cytotoxicity3,5.
Terapias génicas Basado en los sistemas CRISPR-Cas actuales, tiene un uso clínico limitado debido a las frecuentes ocurrencias de edición fuera del objetivo, mutaciones inesperadas de ADN, incluidas grandes deleciones de fragmentos de ADN y grandes variantes estructurales de ADN tanto en sitios dentro como fuera del objetivo que conducen a la muerte celular. y otros problemas hereditarios.
Craspasa (o caspasa guiada por CRISPR)
Researchers have recently reported a novel CRISPER-Cas system which is a Class 2 Type III-E Cas7-11 system associated with a caspase-like proteína hence named Caspasa guiada por Craspase o CRISPR 5 (Caspases are cysteine proteases that play key role in apoptosis in breaking down cellular structures). It has potential applications in areas like gene therapy and diagnostics. Craspase is RNA-guided and RNA-targeted and do not get involved with the DNA sequences. It can target and destroy mRNA and proteínas associated with different genetic diseases more accurately without off-target impact. Thus, elimination of genes associated with diseases is possible by cleavage at mRNA or protein level. Also, when linked with specific enzyme, Craspase can also be used to modify functions of proteins. When its RNase and protease functions are removed, Craspase becomes deactivated (dCraspase). It has no cutting function but binds with RNA and protein sequences. Therefore, dCraspase can be used in diagnostics and imaging to monitor and diagnose diseases or viruses.
Craspase is the first CRISPR-Cas system that shows protein editing function. It is also the first system that can edit both RNA and protein. Its edición de genes function comes at minimal off-target effects and no inheritable problems. Hence, Craspase is likely to be safer in clinical use and therapeutics than other currently available CRISPR- Cas systems 4,5.
Debido a que Craspase supera muchas limitaciones de los sistemas CRISPR-Cas existentes, tiene potencial para revolucionar la terapia génica, el diagnóstico y el control, la investigación biomédica y la mejora de cultivos. Se necesita más investigación para desarrollar un sistema de administración fiable que se dirija con precisión a los genes que causan enfermedades en las células antes de demostrar la seguridad y la eficacia en los ensayos clínicos.
***
Referencias:
- Gostimskaya, I. CRISPR–Cas9: una historia de su descubrimiento y consideraciones éticas de su uso en la edición del genoma. Bioquímica Moscú 87, 777–788 (2022). https://doi.org/10.1134/S0006297922080090
- chao li et al 2022. Herramientas y recursos computacionales para la edición del genoma CRISPR/Cas. Genómica, Proteómica y Bioinformática. Disponible en línea el 24 de marzo de 2022. DOI: https://doi.org/10.1016/j.gpb.2022.02.006
- van Beljouw, SPB, Sanders, J., Rodríguez-Molina, A. et al. Sistemas CRISPR-Cas dirigidos a ARN. Nat Rev Microbiol 21, 21–34 (2023). https://doi.org/10.1038/s41579-022-00793-y
- chunyi hu et al 2022. Craspase es una proteasa activada por ARN guiada por ARN CRISPR. Ciencia. 25 de agosto de 2022. Vol 377, número 6612. págs. 1278-1285. DOI: https://doi.org/10.1126/science.add5064
- Huo, G., Shepherd, J. y Pan, X. Craspase: un nuevo editor de genes duales CRISPR/Cas. Genómica funcional e integradora 23, 98 (2023). Publicado: 23 de marzo de 2023. DOI: https://doi.org/10.1007/s10142-023-01024-0
***